As an electrolyte thermodynamics company, OLI is keenly interested in understanding the challenges that temperature, pressure, and composition bring into reaction and separation processes. A notable affected process is lithium extraction from heavy brines using solar evaporation. Solar evaporation of Andean salars has existed since the mid 1980’s[1]. Heavy brines, including geothermal fluids like the Salton Sea and oil and gas production brines like the Smackover are also being exploited. These brines are also saline but differ in composition and their production locations have evaporative conditions. In this blog post, we explored the thermodynamic outcome of producing an ~8,000-10,000 ppm lithium concentrates from these brines using solar evaporation.
Lithium Concentration and Salt Deposition in the Andes
There are dozens of salt flats in the Andean region. Their common characteristic is the salt-saturated interstitial fluids and the relative abundance of Li, K, and B. We will focus on Salar de Hombre Muerto to study the evaporation process. The major speciesin this salar are Li, Na, K, Mg, Ca, Cl, SO4, and BOH3. The Li, K, and B(OH)3 concentrations in the evaluated sample are ~750, 5500, and 2500 ppm, respectively.
If our process objective is to produce a LiCl concentrate of 50,000 ppm (~8200 ppm Li), then we need to concentrate this brine by ~11x, equivalent to a volume reduction of ~91%. These salars exist at high altitudes in the Andean mountains, and the climate is amenable to their evaporation. The average annual climate parameters include the following[2],[3]: 15oC, 24% relative humidity, 190 mm total rainfall, 6.3 kW/m2 solar radiation, and 4-6 mm/day evapotranspiration rate. Of note, the low pond temperatures promote salt hydrate precipitation.
The following element sequence reflects the precipitation progression when Salar de Hombre Muerto is evaporated to 91% in six fractional steps:
Ca, SO4 > K, Ca, SO4 > K, Ca, SO4, BOH3 > K, SO4, BOH4 > K, SO4, BOH3 > K, SO4, BOH3.
Na and Cl are excluded since halite forms in all stages. The primary minerals are computed to be halite, anhydrite, gorgeyite, syngentite, and boric acid.
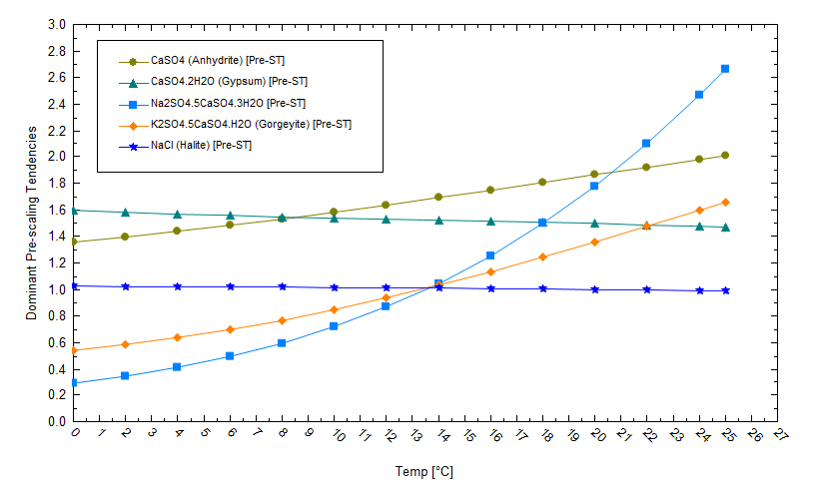
Pond temperatures impact salt phases and solubility differently, and the plot below shows the computed saturation[4] of five salts as temperature increases from 0 to 25oC. A saturation value of 1 means the salt and water are at equilibrium. Values of S>1 indicate supersaturation, conditions under which solids precipitate. NaCl is saturated (S=1) at 15oC and slightly supersaturated at 0oC, but the solubility changes are small given that NaCl solubility has a weak temperature dependence. By comparison, the solubilities of the double salts of Na2SO4.5CaSO4 and K2SO4.5CaSO4 have stronger temperature dependencies and are less soluble at higher temperatures. Therefore, so long as the supply of cations in solution is not limited, the salts that form will depend on the pond temperature. This is important because the calcium concentration is about 1/100th the potassium concentration, and the sulfate concentration is1/15th the chloride concentration. So, there is a combined thermodynamic and mass balance driver to salt deposition. In practice, this means that operators can manipulate conditions to remove materials like calcium, but there may not be much calcium to remove in the first place.
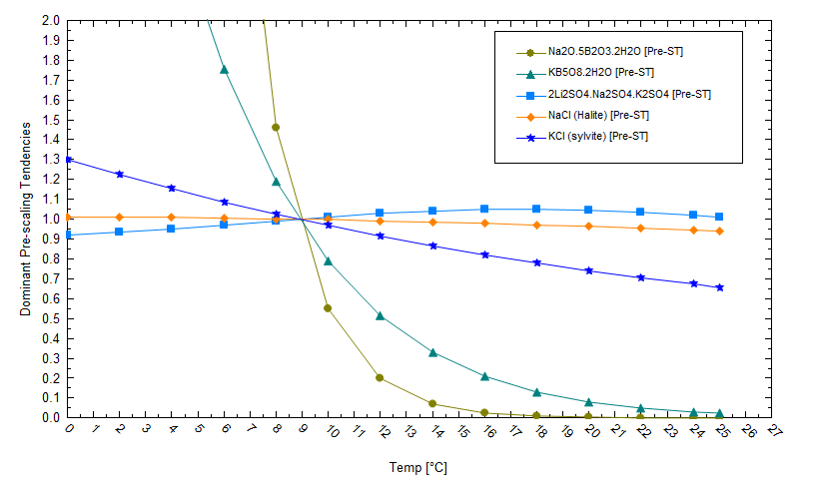
We also need to evaluate the salt saturation condition at the end of the evaporation process. The following plot shows the solubility of several salts in a brine that has evaporated by 91%. If the pond temperature decreases, then lithium becomes more soluble (its saturation values decreases). This demonstrates that lower pond temperatures keep lithium in solution and cause K and Na to drop out as chloride or borate salts. If we reduce the temperature, K and Na will precipitate out as chloride and borate salts, rather than as lithium salts. This will allow lithium to remain soluble to higher evaporation levels. Thus, in this specific example, manipulating temperatures can keep lithium in solution to higher evaporation levels.
The downside of lower operating temperatures is reduced evapotranspiration. We compute that the air mass required to evaporate a 5oC brine to 91% is about 2.6x greater than the air mass required to evaporate a 20oC brine. (The air’s relative humidity is fixed at 40% in both cases.) So, there are practical thermodynamic constraints related to pond temperature, evaporation extent, and salt solubility.
Lithium Extraction in Salton Sea Geothermal Brines
Geothermal brines have varied salt saturation and production temperatures depending on the reservoir chemistry and temperature. For example, brine produced from the Salton Sea geothermal field contains a “dog’s dinner” of metals including high concentrations of Sr, Ba, Fe, Mn, Zn, Pb, and also SiO2 due to high temperatures. Salton Sea brines have around ~200 ppm Li+, in contrast to Andean salars which have Li+ concentrations up to a few thousand ppm. Therefore, if the objective is a 5% LiCl concentrate, then the brine needs to be evaporated to 97.5%. The Salton Sea climate is evaporative, like the Andean salars, so it is thermodynamically feasible to evaporate these brines to dryness. (The practical and environmental feasibilities are an entirely different questions). The following are some climate parameters in the Salton Sea area: 23oC, 29% relative humidity, 100 mm total rainfall, and 6.0 k2/m2 solar radiation. An example evapotranspiration rate is ~5 mm/d [5].
The following sequence demonstrates the order of salts produced when the brine volume is reduced by 97.5% (40x concentration increase) at 30oC:
(FeOH3-Ca-Ni-Zn-Pb-As-B) : Ca, Ba, SO4, CO3, F > Ca, Ba, SO4, F, SiO2 > K, Ba, SO4, SiO2 > K, Ba, SiO2 > K, Ca, Ba, SO4, BOH3, SiO2 > K, Mg, Ca, Sr, Ba, BOH3, SiO2.
The prevalent minerals differ from the Andean salars: halite, sylvite, silica, borates, and barium chloride hydrates.
The first part of the precipitation sequence is chemical treatment and not evaporation. Reduced iron is oxidized to Fe+3 when air contacts the brine. Fe+3 forms Fe(OH)3 flocs, which adsorb F, AsO4, and the other ions listed in the previous paragraph. The net effect is that most of the transition metals are computed to be removed before evaporation begins. Fe(OH)3 precipitation also causes the pH to drop, though this can be managed by adding Ca(OH)2 to the system. Additionally, since Fe(OH)3 forms high surface area flocs, there are additional challenges to dewatering the sludge to minimize lithium losses. The plot below shows the equilibrium REDOX reactions when 1L of Salton Sea brine reacts with up to 10 L air. As air is added, Fe(OH)3 forms, causing the pH to drop to below 2. This acidic condition limits precipitation of other metals such as Ni, Zn, Pb, and Al.
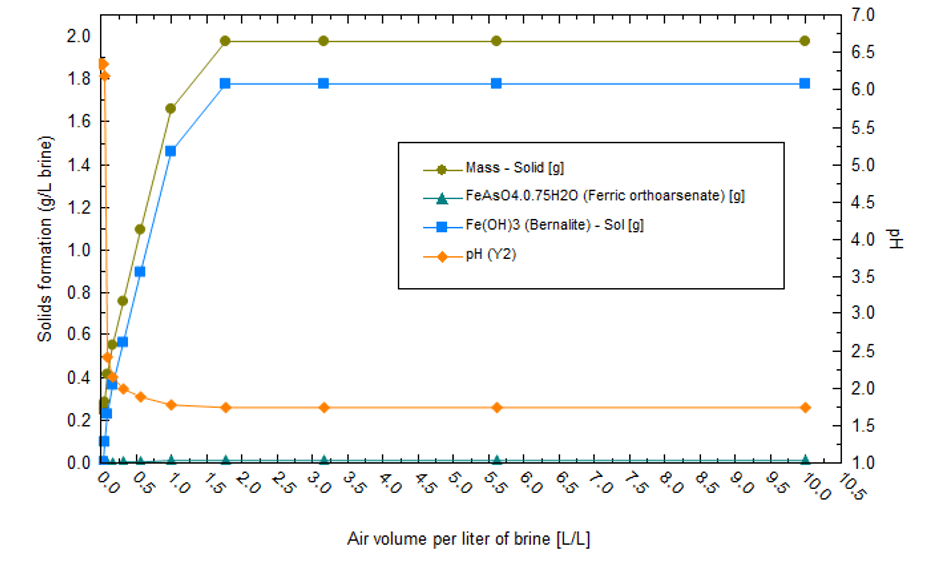
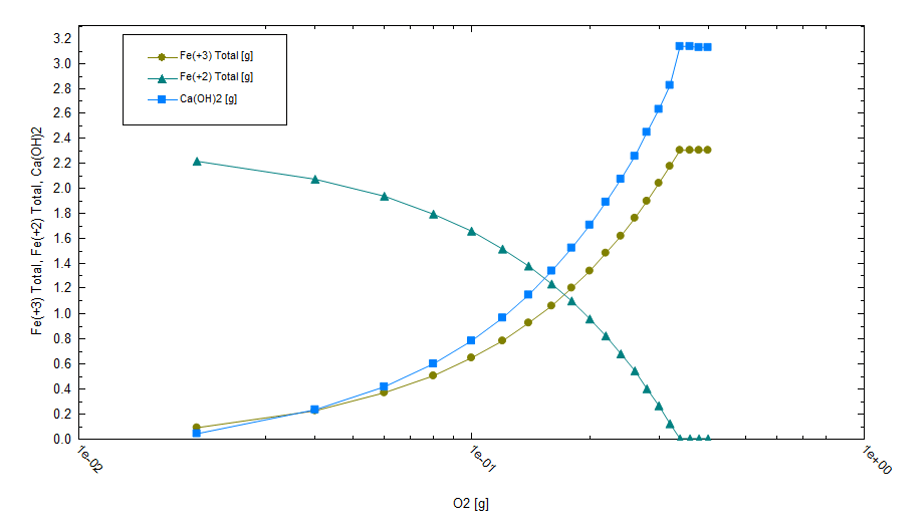
The amount of lime required to maintain pH is proportional to the mass of Fe(OH)3 that forms. From the following plot, we can see that the amount of lime required is nontrivial; about 1.3 g lime must be added per gram of Fe+2 present in the initial brine.
The remaining evaporation process is dominated by K, Sr, and Ba salts of chloride. And, since the brine initially NaCl-saturated, halite precipitates throughout the evaporation process.
Lithium Sequential Evaporation and Mineral Formation in Arkansas Oil and Gas Fields
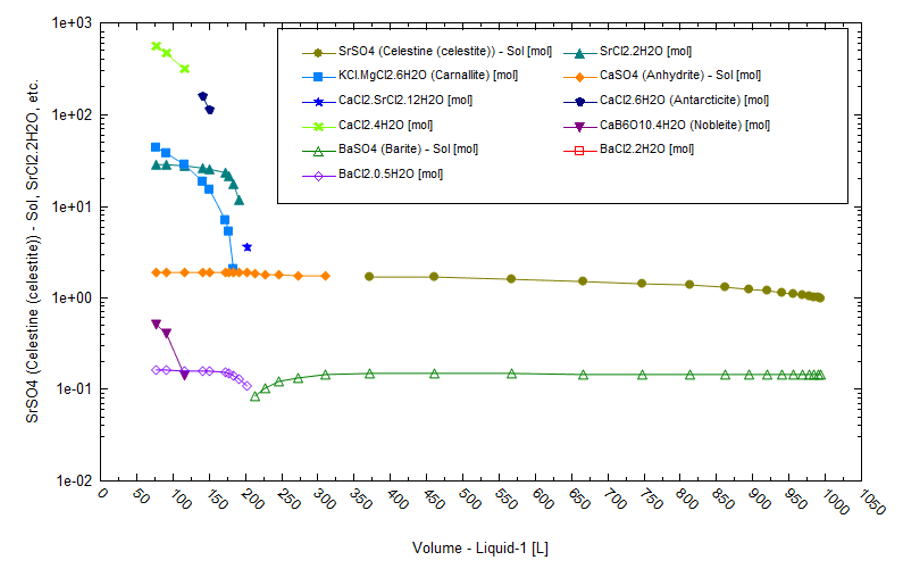
Lithium can also be sourced from brines from oil- and gas-producing fields. For instance, the Smackover formation in Arkansas is currently being exploited for Br and Li. Smackover brines also contain high Na and Cl concentrations but are not necessarily salt-saturated. Instead, the common oilfield scales are saturated minerals such as CaCO3 CaSO4, SrSO4, and BaSO4. We pulled data from a 1974 US Bureau of Mines report[6] for a produced brine in Colombia county. It contained 277 mg/L of Li . The brine is high in K, Mg, Ca, Sr, NH4, and Br when compared to the standard seawater from which it is derived. If this brine were fractionally evaporated to 97% in six stages, then the sequence of elements would be as follows:
Sr, Ba, Fe+3, SO4 > Sr, Ba, SO4 > Ca, SO4 > K, Mg, Ca, Sr, Ba, SO4, BOH3 > K, Mg, Ca, Sr, Ba, SO4, BOH3 > K, Mg, Ca, Sr, Ba, SO4.
The solid phases include halite, barite, celestine, Fe(OH3), CaSO4, carnallite, calcium-barium-strontium chlorides, and calcium borate.
There is little likelihood of any solar evaporation in Arkansas because the region’s climate is non-evaporative. The calculations shown are hypothetical in nature. The following is a list of average climate parameter values: 18oC, 73% relative humidity, 1100 mm total rainfall, and 4.9 kW/m2 solar radiation. The plot below shows the solids that form when the Smackover brine is evaporated to 97 vol% in a single batch at 25oC and low relative humidity. The oilfield scales (BaSO4, SrSO4, and CaSO4) and NaCl (not shown) are the first solids to form. As the liquid evaporates by more than 80%, chloride salts dominate.
High-Salinity Brines and Evolving Technologies
In summary, there are several types of high salinity brines that contain commercial quantities of lithium. In this blog, we looked at three types: an Andean salar, a geothermal brine, and a produced brine. Their general chemistry is consistent, with high concentrations of Na, K, Mg, Ca, and Cl. Their cation ratios and transition metal concentrations vary. The evaporation process for each of these brines produces a similar salt sequence, but with unique complications. The geothermal brine contains high concentrations of reduced iron that will oxidize when in contact with air. Oilfield brines may be amenable to evaporation, but the local climate may render this impractical. Since there are new approaches to extract lithium from these brines, these evaporation techniques are not essential. Lithium from both the Salton Sea and the Smackover brines is being removed with ion-sieve media, a process referred to as “direct lithium extraction,” or DLE. As this technology improves, its applicability to all types of brines will grow, and possibly obviate the need to develop large-area evaporation ponds.
References
[1] https://pubs.usgs.gov/of/1988/0210/report.pdf
[2] weatherspark.com, weatherwx.com, and https://pubs.usgs.gov/of/1988/0210/report.pdf
[3] https://www.lithiumamericas.com/_resources/pdf/investors/technical-reports/cauchari-olaroz/LAC43101Nov112019FINAL.pdf
[4] OLI uses Scale Tendency to describe solubility as shown in the plot
[5] https://www.usbr.gov/lc/region/g4000/4200Rpts/LCRASRpt/2010/report10.pdf
[6] https://www.geology.arkansas.gov/docs/pdf/minerals/USBM_RI-7897_Geochem_Liq_Gas_Rocks_Smackover_FM_.pdf

